Posted by: Eye Centers of Florida in Macular Degeneration
Wet AMD – Macular Degeneration Study
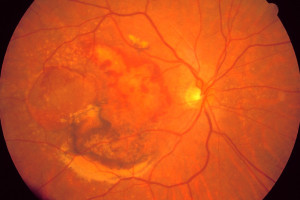
Are you or is someone you love learning to cope with a recent diagnosis of Neovascular Age-related Macular Degeneration, commonly called wet AMD?
If so, participating in our medical research study may be a good option.
Qualified Participants Will Receive
- Study-related care and study medication or placebo at no cost
- Compensation for time and travel
See if You Qualify
Contact us to learn more about participating in our research study of an investigational eye-drop medication for people with wet AMD.
What is Wet AMD?
Wet AMD occurs in about 10% of macular degeneration cases. Sometimes, dry AMD will progress to the wet form. If abnormal blood vessels below your retina start to leak fluid or blood, it can cause rapid and severe vision loss. If you have dry AMD, it’s important to monitor your vision closely for signs of wet AMD. Read more about macular degeneration.
Detailed Description
A Phase 3 Study of the Efficacy and Safety of Squalamine Lactate Ophthalmic Solution 0.2% Twice Daily in Subjects with Neovascular Age-Related Macular Degeneration. Patients will receive injections of ranibizumab. In addition, patients will receive either squalamine lactate 0.2% eye drops or placebo eye drops. The study duration is 2 years.
Phase 3 Study of the Efficacy and Safety of Squalamine Lactate Ophthalmic Solution 0.2% Twice Daily in Subjects with Neovascular Age-Related Macular Degeneration Methodology: Phase III, multicenter, randomized, double-masked, placebo-controlled study conducted in 2 periods (Year 1 and Year 2).
Year 1 (Screening/Baseline to Week 52): Patients will be randomly assigned to one of 2 treatment groups in a 1:1 ratio:
- Squalamine lactate ophthalmic solution, 0.2% BID (Years 1 and 2) + ranibizumab every 4 weeks (Year 1) and PRN ranibizumab (as needed, Year 2).
- Placebo ophthalmic solution BID (Years 1 and 2) + monthly ranibizumab every 4 weeks (Year 1) and PRN ranibizumab (Year 2), based on optical coherence tomography (OCT)-guided re-treatment criteria.